RESEARCH: The "R" in ZRT
Research is at the heart of everything we do. We are actively involved in over 60 research collaborations in 5 countries. These collaborations are primarily with academic institutions and include some with NIH funding, while several are with research organizations or hospitals, and the Centers for Disease Control.
The simple, minimally-invasive and innovative testing methods that are the hallmark of ZRT came about as a result of years of careful research and validation. Through our ongoing research efforts, the methods we use are continually being validated and ZRT Laboratory remains at the forefront of research in all the areas related to our commercial testing:
- Estrogen metabolism
- Urinary elements and environmental toxins
- Hormones and sports medicine
- Menstrual cycle hormonal patterns
- Stress
- Cardiometabolic risk
View our published research papers and a list of abstracts and posters presented at scientific meetings.
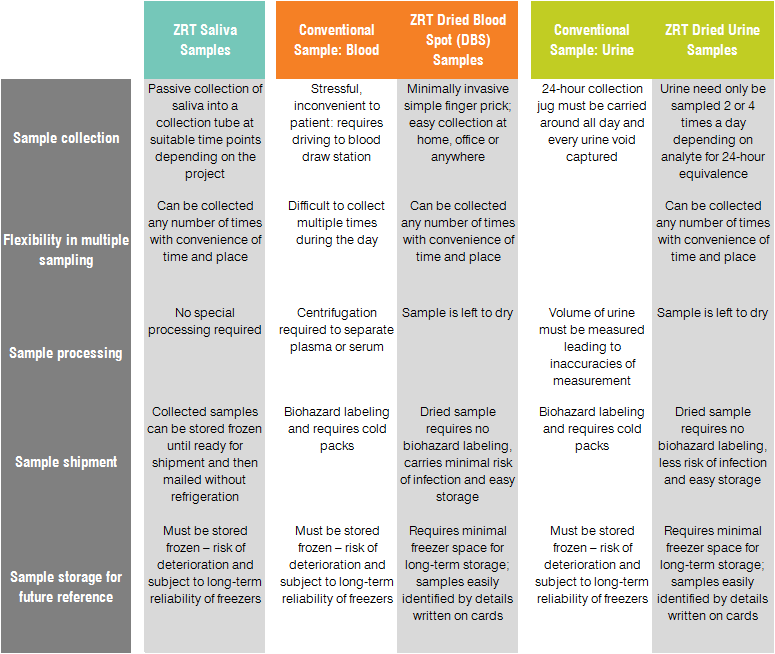
Research sample types that we test include saliva, serum, capillary blood collected via finger stick (dried blood spot, DBS), and urine. The range of testing options we have developed is suited to research applications because samples are easy to collect, store, and ship for testing, and our results are highly accurate. Because collected samples are stable for weeks (saliva) or months (DBS and dried urine) and do not need to be shipped frozen, research can be carried out even in remote areas and samples shipped via regular mail.
The table below summarizes the main advantages of our sample collection methods from a research perspective:
We invite collaborations with clinicians involved in research, including partnerships in clinical trials that require a CLIA-certified testing laboratory for analyses. We provide sample collection materials for saliva, dried blood spot, or dried urine samples. Research samples are tested at ZRT by state-of-the art methodology, including FDA-approved immunoassays, enzymatic assays, inductively-coupled plasma mass spectrometry (ICP-MS), gas chromatography tandem mass spectrometry (GC-MS/MS), and liquid chromatography tandem mass spectrometry (LC-MS/MS).
View technical specifications and validation data for our tests.
Research FAQ's:
For research, we generally supply bulk collection materials at no charge, but you can pay for pre-assembled kits if desired. Outlined below is how we generally handle research samples:
- We ask for a copy of IRB approval for our file, along with a brief summary of the project.
- All sample collection supplies are sent to you at no charge in bulk (not individual kits). You either assist in participant sample collection, or you assemble kits for participants and distribute them as needed for home collection.
- Your participants return their samples to you, and you ship them to ZRT, preferably in batches of 50 or more samples. Shipping costs for returning samples to ZRT are the researcher’s responsibility.
- Test results are provided on an Excel spreadsheet.
Turnaround time for research assays is usually 2-4 weeks, depending on the quantity of samples and method of testing.
We discount pricing for research testing that is approved by an IRB. Pricing is based on volume of testing and can be provided by the ZRT research team if you provide the following information about your study: sample type, analytes of interest, and expected volume (number of research participants and sample collection timepoints).
No, ZRT requires IRB approval for research testing and we ask for a copy for our file. If you would like to test with us without an IRB, a physician can order our clinical testing. Please contact Customer Service about clinical testing by email: info@zrtlab.com or phone: 1-866-600-1636.
The method of testing depends on the analyte and sample type. We test saliva, dried blood spot, dried urine, and serum samples using immunoassays, enzymatic assays, inductively-coupled plasma mass spectrometry (ICP-MS), gas chromatography tandem mass spectrometry (GC-MS/MS), and liquid chromatography tandem mass spectrometry (LC-MS/MS). More information about sample type, testing method, and analyte options can be found on our website in the Test Directory.
Saliva:
Unless shipped within 24 hours of collection, saliva should be stored in a -20°C freezer for up to 1 year until ready to be shipped. If stored for over 1 year, then it should be stored in a -80°C Ultra Low Temp freezer.
Dried Urine and Dried Blood Spot:
Dried urine and dried blood spots must be thoroughly dried (up to 6 hours for blood spots or overnight for dried urine), placed in a baggie with desiccant and sealed, and then either shipped to ZRT within 7 days or placed in a freezer (-20°C for up to 1 year). If storing longer than 1 year, then samples should be placed in a -80°C Ultra Low Temp freezer in a plastic baggie with desiccant.
Serum:
Please reach out to our research team for information on storing participant's serum samples.
We request that you ship research samples to us in bulk (anywhere from 50 or more samples at a time). Saliva, dried blood spot, and dried urine specimens are considered non-hazardous “Exempt Human Specimens” and are not subject to regulation as hazardous materials. However, they must be triple packaged as follows:
Saliva:
The primary container is the saliva tube. Make sure tubes are tightly sealed shut. Secondary packaging must be leak-proof such as a sealed plastic baggie. Saliva shipments also require absorbent material to contain the contents should it leak (paper towels work fine). The third level of packaging must be a rigid container such as a cardboard box to protect the primary and secondary packaging. The shipping container must be labeled with “Exempt Human Specimen.” Saliva can be shipped on dry ice or ice packs using overnight or 2-day shipping methods. Please contact your shipping carrier directly for shipping rates.
Dried Urine & Dried Blood Spot:
The primary container is the filter card itself for dried blood spot or dried urine. We recommend storing them in a sealed plastic baggie with desiccant, which can also be used as the secondary container. The third level of packaging must be a rigid container such as a cardboard box to protect the primary and secondary packaging. The shipping container must be labeled with “Exempt Human Specimen.” Dried blood spot and dried urine cards can be shipped with or without ice packs using overnight or 2-day shipping methods. Please contact your shipping carrier directly for shipping rates.
Serum:
Please reach out to our research team for information on shipping serum samples.
ZRT is committed to protecting research participant confidentiality. For IRB-approved research studies, we only accept de-identified samples, which can be any alpha-numeric code to represent a participant. Results for samples are reported by the participant ID. In the rare case that a research sample is received with identifying information, ZRT employs secure methods to communicate the identity of the research participant with the researcher to assign a de-identified code.
If you have obtained HIPAA Authorization from your research participants, ZRT is a HIPAA covered entity and can comply with privacy standards for research participant PHI.
We report research results on an Excel spreadsheet. We can provide a detailed test report if desired, although there may be more information required from participants to generate the report, such as age and date of last menses for Menstrual Cycle Mapping.
We generally provide research results within 2-4 weeks but can do our best to meet researcher’s timelines when requested. Some types of testing take longer than others, but results are usually reported anywhere from 1-3 weeks at the most.
Yes, we have worked on many projects that require secure transfer of information. ZRT can set up a ShareFile folder to securely send results and other documents. The ZRT research team has also used other methods of secure file transfer and would be happy to meet the requirements of your project.
We can usually test samples that have already been collected, depending on the sample type and analyte. Please see below for specific requirements for each sample type:
Saliva:
Saliva must be collected in a polypropylene tube or glass vial and properly stored (-20°C or -80°C). We require 1.5-2mL of saliva to test. Please inquire with the research team if you have less volume than that and we can discuss options.
Dried Blood Spot:
Blood spot samples must be collected on Whatman® 903 and properly stored (-20°C or -80°C with desiccant. Please inquire with the ZRT research team if you would like us to test blood spot samples collected on Ahlstrom® 226 filter paper as there are multiple factors that determine our ability to do so. ZRT frequently tests banked newborn blood spots successfully. Blood spot samples have demonstrated excellent stability, up to 20 years when properly stored without loss of analyte.
Dried Urine:
We have worked with many researchers who have collected wet urine and stored it frozen (-20°C or -80°C), some for up to a decade. We can either ship urine cards for the researcher to saturate with urine or the researcher can ship us frozen urine for us to saturate the urine cards at our laboratory prior to testing.
Serum:
Please reach out to our research team for more information on previously collected serum samples.
We keep all research samples properly stored for the duration of your research project. Once the findings have been published, we can either ship the samples back to the researcher at their expense or properly dispose of them. There are times a researcher will request additional testing, and we can work with the researcher to do so. We can also hold research samples indefinitely, but there may be an additional charge depending on the request.
An issue we come across frequently is collection date and time on the sample not matching what the researcher reported to us on the Shipping Template. To avoid result delays, please clearly indicate collection date and time on the sample itself and the shipping template. This allows us to validate the information and notify you of any discrepancies.
Insufficient or improperly collected samples seem to be a problem we come across periodically. You can review proper specimen collection methods on our instructional videos on How to Collect Your Sample. Please see below for the issues we commonly see with each sample type:
Saliva:
Saliva samples periodically arrive at the lab without enough volume for us to test. We require 1.5-2mL at the minimum, although we do ask that the saliva tubes be filled at least half full. Other times the caps on the saliva tubes are not properly sealed and when we unpackage them, the saliva has leaked out and there is not enough volume for us to test. Please make sure the tube caps are properly closed.
Dried Blood Spot:
Problems we see with dried blood spot testing are insufficient spots, overlapping spots, blood not soaking through the back of the filter paper, and spot size too small. We prefer you collect a single full blood drop on each of the 12 circles. This will provide adequate dried blood spots for multiple analyte testing, allow for repeat testing if necessary, and provide a dried blood source for additional testing if so requested. Rule-of-thumb is that one full blood drop is adequate for 2-3 tests, but this depends on what type of assay is used for testing. A Blood Spot Reference Information sheet is available that outlines spot size, how many spots are required, and shows pictures of poor and good quality blood spots.
Dried Urine:
We sometimes receive dried urine cards that are still wet usually at the bottom of the card where the filter card is tucked into the cover sleeve. We have not found that this interferes with the analytes we test since they are mostly stable glucuronide and sulfate conjugates of hormones. To avoid receiving slightly moist samples, we ask that you leave the urine strips out overnight to fully dry before storing them in a plastic baggie with desiccant.
We do our best to determine results from a specimen when we can. If a sample is insufficient and not able to be tested, we will not bill for that sample. However, if a specimen is sufficient to test some but not all analytes in a panel, we still charge for the whole panel. Please reach out to the ZRT research team with any specific questions about sufficient samples and billing.
Serum:
Please reach out to our research team for information on testing serum samples.
Yes, we have tested dried blood spots, dried urine, and serum in many different animal species. We have tested reproductive hormones in mammals including cats, dogs, horses, bats, zoo animals, and laboratory mice and rats.

