ZRT Testing Upgrade


A New, Modernized Testing Menu is Here.
A Letter From Dr. David Zava

What's New
Modernization Highlights:
-
Conversion from enzyme immunoassay (EIA) to highly sensitive and specific mass spectrometry and electrochemiluminescence assays
-
Expanded panels and testing options
-
Simplified and descriptive panel names
-
Reduced sample volume requirements for saliva and dried blood spot tests - less sample, more tests
-
Increase in automation to ensure faster turn-around-times
-
Introduction of SKU numbers to make panel identification fasterand more efficient
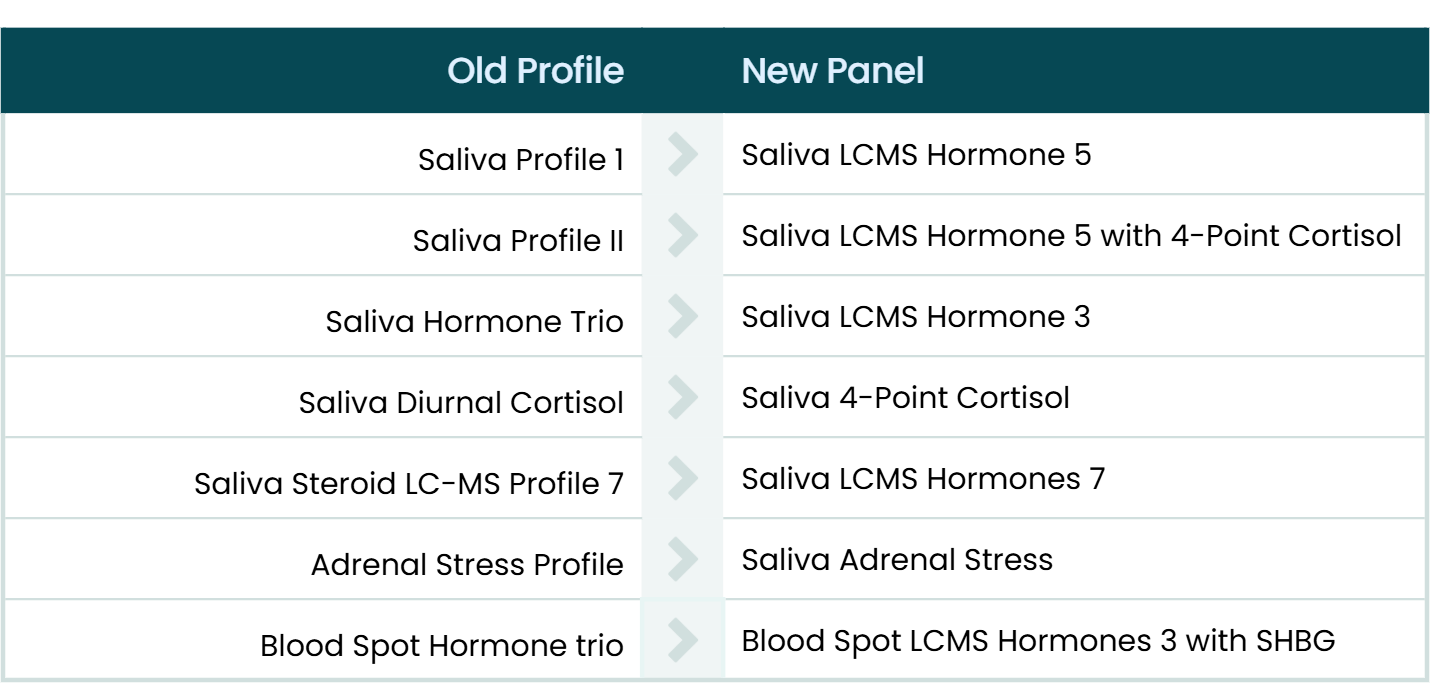
Panel Transition Guide

Resource Links:
How to Access Your Price List on myZRT.com
FAQ
The updated panels and pricing are now live as of January 15, 2026. All new orders placed on or after this date will automatically use the new panels and pricing. We expect a full transition to new panels and methodology by the end of February 2026.
Visit MyZRT.com, log in, and navigate to “Account > Payment Information > View Price List.”
Watch the short tutorial video on this page for step-by-step instructions. New panels can be seen at the bottom of your current price list in the addendum.
Most panels now cost less thanks to a combination of advanced automated electrochemiluminescence technology for things like thyroid and adrenal testing while still utilizing mass spectrometry for complex hormone analysis. A few panels have modest increases due to expanded analyte coverage and inefficiencies caused by low count analyte panels. The best value will be found by combining panels and methodology.
If you need help matching old profiles to our new panels, take a look at the Panel Transition Guide. You can find a full list of new and updated profiles in the Overview of Kits, and all analytes with their assay types in the Directory of Tests.
If you have existing kits, we encourage you to use them prior to the transition on January 15, 2026. All new panels can be run using materials in actively circulating kits. You may be eligible for a kit upgrade at no additional cost for a limited time during the transition window.
Enzyme Immunoassay (EIA) – antibody/antigen-based technology with moderate specificity and sensitivity, low cost, not ideal for complex hormone analysis. Utilized for over a half century. Typically used on 96-well plate hormone assays.
Electrochemiluminescence (ECLIA) – antibody/antigen-based technology with improved specificity and sensitivity, low cost, works well for testing endogenous steroid hormones, but not for testing topical hormone therapies (gross underestimation of hormone delivery to tissues). First utilized at scale in the early 2000s and now commonly used by reference laboratories for automated serum hormone analysis.
Mass spectrometry (LCMS, GCMS, ICPMS) – utilizes different sample preparations to measure analytes, or fragments of analytes, via a mass/charge ratio. Gold standard for high sensitivity/specificity, extended linear range, and complex hormone analysis. Slow adoption due to cost of instrumentation and need for highly trained staff.
Yes and no — increased specificity of mass spectrometry testing means that interferences are greatly reduced compared to enzyme immunoassays. While the analyte itself doesn’t change, those taking supplements or hormones that can cause interference are going to see more accurate results with mass spectrometry without the false hormone signal often seen with steroid hormone immunoassays.
Below is an example of true clinical patient samples run via liquid chromatography mass spectrometry (LCMS) and enzyme immunoassay (EIA) for Progesterone. Significant interference on progesterone was present on 1/3 of samples.

Mass spectrometry provides linear results over multiple orders of magnitude without dilution. Unlike enzyme immunoassays, mass spectrometry is not only sensitive (how low can you go), but retest rates due to results outside of the analytical range are greatly reduced, improving accuracy and turn-around-times.
Most ranges are going to be very similar between ZRT’s current and new testing methods especially when endogenous hormones are being measured. These ranges become more challenging when exogenous steroid hormones are used by different routes of administration (e.g. oral, topical, patch, injections, pellets, etc). ZRT has established ranges for those using hormones based on dose, delivery, and timing from last use. Ranges are laboratory and instrumentation dependent, and it is essential that ranges align with results produced. Introduction of advanced methodology typically justifies a range change. In certain cases, like cortisol analysis, strong linear correlations between methods allow old results to be converted using a simple factor to fit within new ranges. We’ll go into more details about conditions that can significantly alter ranges based on mode of delivery.
Below is an example of true clinical patient samples run via liquid chromatography mass spectrometry (LCMS) and electrochemiluminescence (ECLIA). While the correlation is incredibly strong, mass spectrometry produces slightly lower results due to a true elimination of interferences that register as a hormone signal with many immunoassays. ZRT’s LCMS cortisol range is slightly lower than the electrochemiluminescence range by about 15%.
ZRT first implemented mass spectrometry testing in the early 2000s for vitamin D. Since then, ZRT has expanded our mass spectrometry testing capabilities to include saliva, blood spot, and dried urine hormone, element, and neurotransmitter testing. ZRT currently manages LCMS, GCMS and ICPMS systems for testing. Nearly all of the research samples ZRT tests for steroid hormones utilize mass spectrometry due to its wide acceptance as the gold standard in the academic sector. You can review our extensive list of publications here.
Our Customer Service team is available at info@zrtlab.com or 866.600.1636.

