According to researchers at Vanderbilt University, bromine should be recognized as the 28th essential element for all species, from fruit flies to humans. Study results were published June 5, 2014 in Cell, and demonstrate that without bromine, collagen type IV molecules will not bond together properly to form the structural proteins of connective tissues, leading to disrupted tissue development. 1
To find out more details, here is a YouTube video explaining their work.
This figure demonstrates bromines role as a co-factor in formation of collagen IV crosslinks:
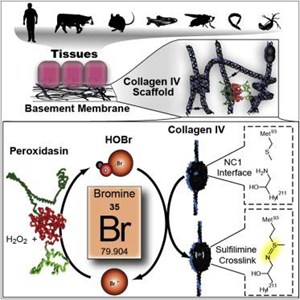
Image Source: McCall S, Cummings C, Bhave G, Vanacore R, Page-MCaw A, Hudson B. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell. 2014;157:1380-92.
At this point it may be premature to say that bromine should be added to the list of elements essential to human life until this work is unequivocally confirmed by other researchers in humans. Nevertheless it is very exciting to hear that bromine is apparently essential to collagen formation in species throughout the phylogenetic spectrum, from insects to humans.
Bromine Background
- Bromine is a halogen and the 35th element on the periodic table, sharing similar elemental properties with iodine, chlorine and fluorine.
- Currently the acceptable daily intake for bromine is 1 mg/kg body weight (50 mg in a 50 kg person). In the United States the typical daily intake is around 2-8mg from grains, nuts and fish. 2 3
- Bromine is toxic at higher levels (intakes >50-100 mg/day). 4 Until now it was unclear if bromine had any essential functions in humans.
- Bromine has been used historically at levels up to 6 grams/day as a sedative, anti-libido or anti-convulsant, and was found in popular medications such as Bromoseltzer. 5 6 7
- Bromine is commonly used in fumigants, flame proofing agents (e.g. Polybrominated diphenyl ethers [PBDE]), water purification, medicines (e.g. Bromide inhalers), swimming pool disinfectants, and popular beverages (e.g. Brominated vegetable oil in Gatorade, Fanta, Mountain Dew).
- The largest reservoir of bromine is the ocean, with levels ranging from 65-80 mg/L. 8 Products high in bromine typically come from the ocean (e.g. fish, shellfish, seaweed)
- Bromine is primarily excreted in urine, so urine values represent intake. 9 10
Bromine Toxicity: Bromism
Bromine toxicity, or bromism, is a result of excessive bromine consumption or exposure. Bromism presents itself as neurological symptoms such as headache, slurred speech, confusion, hallucinations, stupor and coma. 11 Although bromism is rarely seen today, it was common decades ago when bromine-containing medication was readily available. In many cases bromism was misdiagnosed and patients received higher doses of bromine to try and prevent the symptoms caused by bromine, only to make things worse. A serum bromide concentration >500 mg/L (Extremely High!!) is confirmatory for bromism. Symptoms are rarely seen below this level. 12
Human Studies
Very few human studies have been completed, primarily because the effects of bromine at lower levels have yet to be elucidated. In one study 21 healthy volunteers were given 1 mg bromide/kg body weight (the current acceptable daily intake) for 8 weeks, and no significant changes were observed in serum hormone concentrations (thyroid hormones, cortisol, testosterone, estradiol, progesterone ). 13 This study was repeated with concentrations of 0, 4 or 9 mg bromide/kg body weight, and a significant, but within normal range, increase in thyroid hormones was seen in female subjects at the highest supplement level. 14
Rat Studies
Multiple rat studies have shown the toxic effects of bromine at high supplemental levels. It was demonstrated that during periods of iodine deficiency the symptoms of hypothyroidism were enhanced when high levels of bromide were added to the diet. 15 In a similar study, high rat bromide intake resulted in decreased radioiodine accumulation and a reduced half-life of iodine from 92 to 30 hours in the thyroid. This was observed in both rats fed an iodine sufficient and iodine deficient diet. 16 Breast milk iodine transfer in rats was also significantly decreased when bromine intake was increased. 17
Conclusion
Like the essential elements iodine and selenium, new research points to bromine as an essential element in the human body. It will take many years of research to determine the dietary range in which bromine is required. Information on bromine toxicity is readily available, but sufficient and deficient dietary intakes have yet to be determined.
Learn more about ZRT Laboratory's multi-element analysis in dried urine that measures bromine and other essential and toxic elements.
References
- McCall S, Cummings C, Bhave G, Vanacore R, Page-MCaw A, Hudson B. Bromine is an essential trace element for assembly of collagen IV sceffolds in tissue development and architecture. Cell. 2014;157:1380-92.
- FAO/WHO. Pesticides residues in food. Joint report of the FAO Working Party on Pesticide Residues and the WHO Expert Committee on Pesticide Residues. FAO Agricultural Studies, No. 73; WHO Technical Report Series, No. 370.
- Nielsen FH, Dunn M (2009) Bromine. In: Other trace elements. Bethesda, MD, American Society for Nutrition (http://jn.nutrition.org/nutinfo/content/trace.shtml).
- Fact Sheet. Facts About Bromine – Emergency Preparedness and Response Web site, 2007. http://www.bt.cdc.gov/agent/bromine/basics/pdf/factsheet.pdf
- van Leeuwen FX, Sangster B. The toxicology of bromide ion. Crit Rev Toxicol. 1987;18(3):189-213.
- Lyon TDB, Robin PA, Watson WS, Littlejohn D. Determination of elevated concentrations of bromine in serum by ICP-MS and ICP-OES. J Anal At Spectrom. 2005;20:757-9.
- Bromide in drinking-water, Background document for development of WHO Guidelines for Drinking-water Quality, World Health Organization (2009).
- Bromide in drinking-water, Background document for development of WHO Guidelines for Drinking-water Quality, World Health Organization (2009).
- Allain P, Mauras Y, Dougé C, Jaunault L, Delaporte T, Beaugrand C. Determination of iodine and bromine in plasma and urine by inductively coupled plasma mass spectrometry. Analyst. 1990 Jun;115(6):813-5.
- van Leeuwen FX, Sangster B. The toxicology of bromide ion. Crit Rev Toxicol. 1987;18(3):189-213.
- Bromism: In: Parfitt K, ed. Martindale 32nd ed. Pharmaceutical Press, 1999:1620-1623.
- Carney MW. Bromism--a clinical chameleon. Nurs Times. 1973 Jul 5;69(27):859-61.
- Sangster B, Krajnc EI, Loeber JG, Rauws AG, van Logten MJ. Study of sodium bromide in human volunteers, with special emphasis on the endocrine system. Hum Toxicol. 1982 Oct;1(4):393-402.
- Sangster B, Blom JL, Sekhuis VM, Loeber JG, Rauws AG, Koedam JC, Krajnc EI, van Logten MJ. The influence of sodium bromide in man: a study in human volunteers with special emphasis on the endocrine and the central nervous system. Food Chem Toxicol. 1983 Aug;21(4):409-19.
- Buchberger W, Holler W, Winsauer K. Effects of sodium bromide on the biosynthesis of thyroid hormones and brominated/iodinated thyronines. J Trace Elem Electrolytes Health Dis. 1990 Mar;4(1):25-30.
- Pavelka S, Babický A, Vobecký M, Lener J. Effect of high bromide levels in the organism on the biological half-life of iodine in the rat. Biol Trace Elem Res. 2001 Summer;82(1-3):125-32.
- Pavelka S, Babický A, Lener J, Vobecký M. Impact of high bromide intake in the rat dam on iodine transfer to the sucklings. Food Chem Toxicol. 2002 Jul;40(7):1041-5.