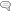As a hormone testing lab, ZRT understands the importance of a well-balanced endocrine system in maintaining overall health.
Hormone balance is achieved through multiple feedback mechanisms, and when any part of the system is thrown out of whack by forces beyond its control, there is a knock-on effect on the rest of the body systems that are under endocrine control.
Such forces can include extreme or chronic stress, or exposure to environmental toxins that enter the body through the air we breathe or in our diets. Substances in the environment that upset the endocrine system are known as endocrine-disrupting chemicals, or EDCs.
How do EDCs cause disruption?
There are 3 main mechanisms by which substances in the environment exert endocrine disrupting effects. First, they can mimic, or act as, a hormone that occurs naturally in the body, activating that hormone’s receptors and often producing inappropriate or excessive hormonal effects. Second, they can block the activity of endogenous hormones by occupying their receptors without activating them. And third, they can interfere with synthesis or metabolism of hormones and/or their receptors, indirectly affecting or modulating the body’s responses to endogenous hormones.
Because of the importance of the endocrine system in early development, some of the greatest risks from EDCs are to pregnant and breastfeeding mothers and to children at important stages of growth and development, in whom endocrine disruption can have very serious long-term consequences including problems with fertility and increased cancer risk when they reach adulthood. But the effects of EDC exposure in adulthood are also implicated in infertility and risks of some cancers.
Those EDCs that interfere with the hypothalamic-pituitary-gonadal axis, notably polychlorinated biphenyls (PCBs), bisphenol A (BPA), and lead, are particularly damaging to the developing reproductive system, and depending on the dose can either delay or hasten puberty, and cause long-term effects on male and female fertility. However, there is little evidence for alterations in endogenous hormone levels as a result of exposure to EDCs.
What are the main culprits?
Estrogenic EDCs are of concern because of their potential to increase risk of hormone-dependent cancers, particularly breast cancer |
Many substances in the environment, both naturally occurring and man-made, have been identified as EDCs. These include pharmaceuticals, agricultural chemicals such as pesticides, plasticizers like BPA, and heavy metals. These substances can be found in plastic materials used for food storage, food itself, cosmetics, detergents, household cleaning products, and flame retardants.
Organizations such as the Environmental Working Group (EWG) have provided materials that can help people know where EDCs occur and avoid them – for example, their annually-updated list of produce items that are most likely to be contaminated with pesticides (the "dirty dozen") for which consumers are advised to buy organic instead, and those that are least likely to be contaminated (the "clean fifteen").
A significant group of EDCs consists of those that have estrogen-like activity. These include naturally occurring phytoestrogens (e.g., genistein), xenoestrogens (non-naturally occurring estrogen mimics like BPA), and the metalloestrogens (heavy metals that can activate the estrogen receptor). Estrogenic EDCs are of concern because of their potential to increase risk of hormone-dependent cancers, particularly breast cancer. Let's look in more detail at these estrogen-mimicking EDCs.
Bisphenol A
BPA is found principally in linings of food and beverage cans and in thermal paper used in receipts. We are mainly exposed to it by consuming canned foods and beverages, and we absorb smaller amounts through the skin when handling cash register receipts or paper money, which is contaminated with BPA through incidental contact with receipts. BPA acts like a weak estrogen in the body, but it isn’t a steroid – it is made from two phenol molecules, each of which consist of a 6-carbon ring with a hydroxyl group attached, combined chemically with acetone; hence the name: bis (2) phenol A (acetone), as shown below.
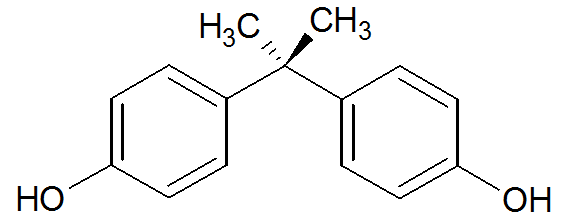
However, its structure is similar enough to estradiol (see the structures below, showing BPA oriented similarly to estradiol) that it can exert endocrine-disrupting effects through its strong binding to estrogen-related receptor γ (ERR-γ) and, more weakly, to estrogen receptors (ER) α and β.
BPA Estradiol
Heavy metals
The metalloestrogens include metal/metalloid anions, e.g., arsenite, and bivalent cations, e.g., cadmium, cobalt, copper, nickel, chromium, lead, mercury, and tin. We are exposed to these metals through the diet, drinking water, and, for cadmium especially, smoking. The bivalent cations can activate the estrogen receptor α (ERα) in the absence of estradiol, putatively by mimicking the binding of calcium to the ligand-binding domain of the receptor [1], while arsenic's interference with ER-mediated gene expression has been less clearly defined and could involve multiple mechanisms [2].
Heavy metals have multiple toxic effects, particularly at high doses, which are not necessarily related to endocrine disruption. For example, exposure to lead is well known to induce male infertility by impacting spermatogenesis, but the mechanisms for this are different depending on the level of exposure. High doses from occupational exposure have direct cellular toxicity in the male reproductive system, while long-term low-level exposure, rather than the more obvious occupational exposure, can be much harder to detect but can result in adverse effects as a result of endocrine disruption. These effects include suppression of hormone synthesis by disrupting signaling in the hypothalamic-pituitary-testosterone axis, adversely affecting testicular function and spermatogenesis [3]. Mercury is a weak estrogen mimic and has been implicated in animal studies in feminization of males and suppression of fertility in females, although as with lead, effects of mercury on reproductive function are different at different levels of exposure [4].
Those metals that can accumulate in the body over many years – notably cadmium, lead, and mercury – are of particular concern. Cadmium’s properties as a metalloestrogen are the best characterized of all the heavy metals [4], and its potential for increasing risk of estrogen-dependent cancers such as breast cancer is compounded by its bio-accumulative properties. Cadmium exposure in non-smokers is mainly from the diet, as foods grown in areas where soil cadmium levels are high can be contaminated, while smokers are at particularly high risk of direct cadmium exposure via inhalation of tobacco smoke.
Be aware of exposure to EDCs
For EDCs like BPA and heavy metals, it’s important to be aware of where these occur, and do what we can to minimize exposure. For example, look for BPA-free cans and plastics for food storage, avoid smoking, and be aware of heavy metals in the environment by paying attention to news of leaks from industrial activity. We can also detect exposure by urine testing; BPA testing is included in ZRT’s hormone metabolite testing, while heavy metals are tested in ZRT’s heavy metal and nutrient profiles.
Related Resources
- Blog: Does Bioaccumulation of Toxic Elements Lead to Large Problems?
- Blog: Are Heavy Metals in Lipstick Making Us Sick?
- Web: Simple Testing for Heavy Metals
- Endocrine Disruptors. National Institute of Environmental Health Sciences (NIEHS).
- Bisphenol A. National Institute of Environmental health Sciences.
References
[1] Byrne C, et al. Metals and Breast Cancer. J Mammary Gland Biol Neoplasia. 2013;18(1):63–73.
[2] Watson WH, et al. Arsenic: Extension of its Endocrine Disruption Potential to Interference with Estrogen Receptor-Mediated Signaling. Toxicol Sci 2007; 98(1):1-4.
[3] Vigeh M, Smith DR, Hsu P-C. How does lead induce male infertility? Iranian Journal of Reproductive Medicine. 2011;9(1):1-8.
[4] Dyer CA. Heavy Metals as Endocrine-Disrupting Chemicals. In: Gore AC, editor. Endrocrine-disrupting chemicals: From basic research to clinical practice. Totowa, NJ: Humana Press Inc; 2007.