While it sometimes seems that babies are everywhere, for many people the process of becoming a parent can be a long and heart-wrenching journey. Infertility affects 1 out of 6 people with one-third of the cases due to female issues, one-third due to male issues, and one-third due to the couple together (1).
It is estimated that approximately 10% of all women have polycystic ovarian syndrome (PCOS) and that around 80% of them will struggle with infertility (2). While many women with PCOS will achieve pregnancy on their own, others will need medical assistance to become pregnant.
In the last couple of years, the research world has dramatically expanded our comprehension of PCOS. It has long been known that PCOS involves elevated levels of female androgens particularly testosterone and DHEA. Newer research has provided deeper knowledge into the interplay between environmental, immunological, inflammatory, hormonal, and genetic factors. This rise of knowledge underscores the reality that PCOS is a more systemic imbalance far beyond the ovaries and presenting a more comprehensive challenge to those it affects. As we delve into the conversation about PCOS and its impact on fertility, it's essential to highlight several recent key insights that have emerged:
- Hormonal Imbalances: Women with PCOS struggle with higher androgens (testosterone and DHEA/S), but research shows variances in hormone receptors may be to blame. This intricate dance of hormones and their movement in and out of cells play a central role in the condition's development and its myriad of symptoms.
- Beyond the Ovaries: While PCOS manifests with ovarian symptoms, it is also a lifelong mental health and cardiometabolic condition. This perspective is crucial, as it emphasizes the importance of a holistic approach to management; recognizing that PCOS affects much more than reproductive health.
- Genetic Variances: The field of DNA research is revealing significant genetic variants present in women with PCOS. These include alterations in the cellular functions of adipose tissue, insulin, melatonin, adrenal function, and androgens (notably testosterone and DHEAS). This genetic backdrop contributes to the condition's complexity and individual variability in symptoms and responses to treatment.
- A Lifelong Journey: It's important to understand that PCOS is a lifelong condition persisting even if the ovaries are removed. This reality highlights the need for ongoing management and support for those affected.
- A Family Affair: PCOS runs in families. Many women with PCOS report having family members who also struggle with the condition reflecting how genetic predispositions play a role in its symptom development.
These insights into PCOS illuminate the condition's multifaceted nature, encouraging a compassionate, informed approach to care and support for those navigating its challenges.
Physiology of PCOS is Complex
Before exploring how PCOS affects fertility, it’s helpful to understand that PCOS embodies a complex physiological condition. In PCOS we find genetic variances in hormone levels, neurochemistry, cell receptor numbers, decreased receptor functionality, and altered hormone metabolism within the cells which all contribute to a variety of patient symptoms. Analogous to cell doorways, receptors serve as entry points for hormones, each requiring a specific key. These "doors" and their mechanisms are inherited and may differ in size, efficiency, and quantity among individuals. In PCOS, receptor dysfunction arises when these keys fail to operate correctly, leading to improper use, recycling, and opening of the receptor "doors."
Many receptors are altered in PCOS including those for norepinephrine, estrogen, vitamin D, adiponectin, cortisol, and testosterone; all which underscores the systemic nature of PCOS. Focusing on the role of insulin, identified as a key factor in approximately 70% of women with PCOS, sheds light on how this condition transcends an isolated ovarian disorder to implicate systemic challenges (3). Insulin levels are generally elevated in women with PCOS independent of weight or carbohydrate intake. Newer research is showing that this is due to anormal insulin receptors and likely abnormal insulin metabolism. Higher insulin then sets off a cascade affecting various hormones including LH, DHEAS, DHEA, androstenedione, and testosterone, contributing to weight gain, abdominal obesity, reduced sex hormone-binding globulin (SHBG), and heightened inflammation. These hormonal imbalances manifest as hallmark symptoms of PCOS, such as:
- Weight-gain especially in the abdomen
- Acne
- Excessive hair growth on the face and body
- Scalp hair loss
- Irregular or absent menstrual cycles
Furthermore, sustained high insulin levels elevate risks for lifelong conditions including:
- Fatty liver disease (NASH/NAFLD)
- High blood pressure (hypertension)
- Insulin resistance
- Diabetes Type 2
High Insulin Impacts Fertility
Elevated insulin levels and higher androgens (testosterone and DHEAS) in PCOS are problematic for fertility. This hormonal landscape often results in a proliferation of immature ovarian cysts, impeding the maturation process of ovum (eggs), and decreasing the progression to ovulation. In scenarios where insulin and testosterone levels are significantly elevated, women may experience challenges in achieving routine ovulation, with some women not experiencing menstrual periods without pharmacological support. On occasions where ovulation is successful, the eggs produced by women with PCOS tend to be of lower maturity, which may affect fertilization, embryo quality, and decrease implantation rates. Despite these challenges, many women with PCOS achieve pregnancy on their own. Others find fertility success through ovulation stimulants or in vitro fertilization (IVF). Treatments such as letrozole or clomiphene are known to elevate gonadotrophin hormones (FSH and LH), fostering the development of more mature ovum. Some women may achieve pregnancy swiftly with these treatments, while others might observe significant benefits from a regimen extending over 3-4 months, which promotes a hormonal environment conducive to healthier egg development.
Semaglutide, GLP1 and Fertility with PCOS
The introduction of GLP1 medications has been notable for individuals with type 2 diabetes, but usage in women with PCOS has shown remarkable weight loss in women who have struggled to lose weight with other medications or regimens. Recently, a flood of reports of “Ozempic babies” has been noted as women have gotten spontaneously pregnant after starting a GLP1 for weight loss including among women who have done IVF unsuccessfully in the past. These occurrences underline the strong negative impact of insulin on fertility even in the face of assisted fertility technologies. Questions remain if the main impact of the GLP1s is to lower overall insulin levels thereby improving egg quality and fertilizations or if the GLP1s have separate action on egg maturity, fertilizations or implantations. What is clear however, is that GLP1s should be considered in women with high insulin levels especially if weight is also an issue, before or as part of a plan to do assisted reproduction.
On "The Pill” for PCOS and Want to Get Pregnant
Many people with PCOS turn to oral contraceptives to regulate menstrual cycles and address symptoms associated with elevated testosterone levels. These medications not only facilitate regular uterine bleeding but also play a significant role in reducing ovarian testosterone production, decreasing adrenal androgens, and fostering an environment rich in estrogen. Additionally, oral contraceptives increase the levels of sex hormone-binding globulin (SHBG) and cortico-binding globulin (CBG), additionally lowering androgens. This control of testosterone effectively alleviates acne, excessive facial and body hair, scalp hair loss, and aids in the maintenance of regular menstrual cycles. Furthermore, for numerous women, the use of oral contraceptives contributes positively to weight management. Although a slight increase in insulin resistance may occur, the overall reduction in androgen levels typically leads to decreased insulin and cortisol levels, and subsequently, weight stability.
However, when the pursuit of fertility becomes a priority, discontinuing oral contraceptives becomes necessary, reintroducing previous challenges. Stopping oral contraceptives prompts the ovaries to resume their natural cycle. While many women are under the belief that they should allow their cycle to happen for several months before attempting pregnancy, the initial 1-2 months post-discontinuation of oral contraceptives may present the highest fertility potential for women with PCOS. This phenomenon contradicts common expectations, as the resumption of natural ovulation commonly and quickly leads to increased testosterone levels which may reduce ovulation frequency and, consequently, reduce the chances of conception. Therefore, for women with PCOS wishing to conceive may wish to attempt pregnancy as soon as possible after discontinuing the contraceptives to optimize fertility opportunities.
Pregnancy and Beyond
Upon achieving pregnancy, it is prudent for women with PCOS to be mindful of the high likelihood of developing gestational diabetes. It is advisable to monitor not only blood sugar levels and hemoglobin A1C but also insulin levels; testing both fasting and those following meals or a glucose challenge. Elevated insulin and glucose levels during pregnancy can lead to various complications, including accelerated growth and increased size of the baby, heightened risk of hypertension in the mother, premature deliveries, neonatal blood sugar complications, and possibly an elevated risk of diabetes in the child later in life. Moreover, increased insulin levels have been implicated in reducing development of breast ducts, potentially complicating lactation post-delivery (4). Embracing a diet rich in protein, low in simple carbohydrates, and abundant in fiber can significantly contribute to maintaining optimal blood sugar and insulin levels throughout pregnancy.
While all of these challenges make pregnancy difficult in women with PCOS, it is important to once again stress that most women with PCOS are able to become pregnant. For providers who are caring for women with PCOS, it is the aim to optimize health for the woman before, during and after pregnancy. With our growing knowledge of this condition, we can appreciate that PCOS is a much more systemic condition than ever before. The next blog on PCOS will look at lab testing and some of the treatments that may wish to be considered.
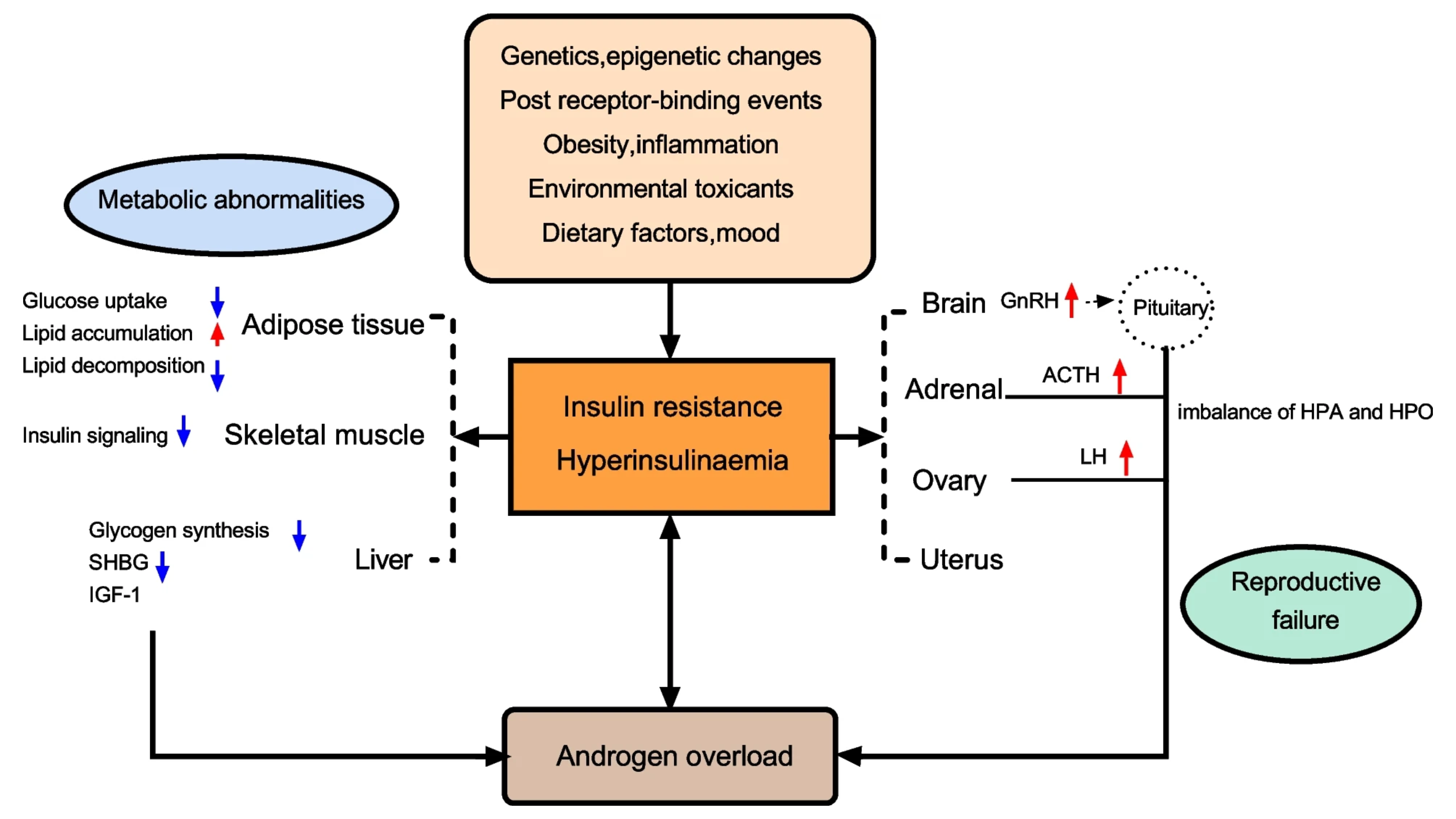

Figure 1. A summary of the most representative impact of IR and HI in women with PCOS. Abbreviations: SHBG: sex hormone-binding globulin; LH: luteinizing hormone; IGF1: insulin growth factor 1; GnRH: gonadotropin-releasing hormone; ACTH: adrenocorticotropic hormone; HPO: Hypothalamus-pituitary-ovary; HPA: Hypothalamus–pituitary–adrenal (5).
References:
- World Health Organization. Handbook on Health Inequality Monitoring: With a Special Focus on Low- and Middle-Income Countries. World Health Organization, 2013, https://www.who.int/publications/i/item/978920068315.
- Melo, Anderson Sanches, Ferriani, Rui Alberto, & Navarro, Paula Andrea. "Treatment of infertility in women with polycystic pvary syndrome: approach to clinical practice." NIH. Clinics, Nov. 2025, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4642490/.
- Legro, Richard S, Castracane, V Daniel, & Kauffman, Robert P. "Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls." NIH. PubMed, Feb. 2004, https://pubmedncbi.nlm.nih.gov/14752392.
- Nommsen-Rivers, Laurie A. "Does Insulin Explain the Relation between Maternal Obesity and Poor Lactation Outcomes? An Overview of the Literature." NIH. Science Direct, Mar. 9, 2016, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4785481.
- Zhao, Han, Zhang, Jiaqi, Cheng, Xiangyi, Nie, & He, Bing, "Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment." Biomed Central. Journal of Ovarian Research, Jan 11, 2023, Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment | Journal of Ovarian Research | Full Text (biomedcentral.com).