Premenstrual Dysphoric Disorder (PMDD) is a premenstrual disorder characterized by physical and psychological symptoms that occur in the luteal phase of the menstrual cycle and are often more extreme than the more common symptoms associated with Premenstrual Syndrome (PMS). PMS affects 20-40% of menstruating women and common symptoms include fatigue, irritability, mood swings, depression, abdominal bloating, breast tenderness, acne, changes in appetite and food cravings. PMDD occurs in 5-8% of menstruating women and is characterized by extreme mood and physical symptoms that interfere with quality of life to a significant degree (1).
Relationships, school, and work can often suffer in the last one or two weeks leading up to the menstrual cycle. Potentially half of a woman’s reproductive years may be spent in a state of serious depression, anxiety, and irritability. Beyond reproduction, hormones are powerful signaling molecules with receptors throughout the body, brain, and nervous system. Estrogen and progesterone have a profound influence on mood, cognitive function, stress tolerance, sleep, and an overall sense of well-being.
In Part II of this examination of PMDD, we will take a closer look at the effects of estrogen, thyroid function, and the hypothalamic-pituitary-adrenal (HPA) axis. The difficulty in regulating emotion is characteristic of PMDD and is related to sex hormone fluctuations and their influence on key areas of the brain that control emotion, memory, and cognition. I will also provide a brief overview of common conventional and complementary treatment considerations along with testing options to identify potential contributors to PMDD.
Depression and anxiety disorders in women
Though this is an article on PMDD, which is classified as a major depressive disorder (MDD) with a temporal relationship to the luteal phase of the menstrual cycle, it is important to acknowledge that there is an increased tendency for women to experience issues with general depression and anxiety two to three times as often as their male counterparts. Several studies point to the relationship of estrogen and its impact on cognitive function, mood, emotional regulation, and stress management (2).
Estrogen and progesterone receptors are highly expressed in areas of the brain involved in emotion and cognition such as the amygdala and the hippocampus (3). Increased vulnerability to depression in women often begins in puberty with a decline in new onset mood disorders after menopause. Perimenopause is a particularly vulnerable time for mood disorders as menstrual cycles become dysregulated (2). Progesterone tends to drop off sharply due to anovulatory cycles and estrogen levels can become erratic with higher highs and lower lows, making menopause a welcome end to the unpredictable hormonal fluctuations.
PMDD has often been described as a heightened sensitivity of the central nervous system to normal variations in ovarian hormones across the menstrual cycle. While ovarian hormones are key to reproductive function, they also regulate neurotransmitter systems within the brain and nervous system. It follows that if hormones regulate neurotransmitter production and receptor sensitivity, the fluctuation of hormones will also affect neurotransmitter systems.
Estrogen and PMDD
The effects of estrogen on mood are well-established. However, both estrogen and progesterone are present in the luteal phase of the menstrual cycle, so it is important to understand the effects of estrogen in relation to progesterone.
The presence of estrogen is necessary to create progesterone receptors and the presence of progesterone downregulates estrogen receptors (4). Both hormones need to be present to optimize and regulate the function of the other. In a 1988 study by Holt et al examining the mechanisms of steroidogenesis in the corpus luteum of rabbits, it was determined that the presence of estrogen was necessary to increase progesterone levels. The mechanism by which this occurred was through estrogen’s effect on the storage of cholesterol and the further processing of cholesterol to pregnenolone (steroidal hormone precursor) in the mitochondria. In estrogen-deprived rabbits, the serum progesterone levels fell precipitously in vivo within 24-hours. In the rabbits with ongoing estrogen stimulation, serum progesterone levels remained high (5).
In a more recent study, Yen et al concluded that single point analysis of hormones in the luteal phase of the cycle does not reveal the hormonal dynamics that may occur throughout the luteal phase of the menstrual cycle. In a more nuanced comparative analysis of hormonal fluctuations in women with PMDD, subtle differences in hormone levels in the early and late luteal phases of the menstrual cycle are revealed. Yen et al evaluated estrogen and progesterone levels in the early luteal (EL) and late luteal (LL) phases of the menstrual cycle amongst 63 women with PMDD and 53 controls (6).
The results revealed that women with PMDD have lower EL-phase and LL-phase estrogen along with a higher level of EL-phase progesterone as compared to controls. The low estrogen and higher progesterone levels in the EL-phase also show an association with LL-phase PMDD severity. It was concluded that EL-phase low estrogen may promote a vulnerability to the effect of progesterone (and its metabolite allopregnanolone) in women with PMDD (6).
Ko et al also revealed that women with higher estrogen in the mid luteal phase experienced less severe PMDD symptoms. These findings were consistent with other studies indicating the potential for estrogen to mitigate stress and depressive symptoms by enhancing cognitive function and protecting hippocampal activity while under stress (7). These effects may also be attributed to the enhanced effect of serotonin in the presence of higher estrogen levels.
In a 2007 study conducted by Huo et al, variants in the estrogen receptor alpha gene (ESR1) are demonstrated in women with PMDD. ESR1 plays a major role in brain stimulation, and dysfunction of this receptor may lead to the cognitive, somatic, and mood changes seen in PMDD. ESR1 also regulates signaling of neurotransmitter systems implicated in both the pathogenesis and treatment of PMDD (8).
Estrogen affects multiple neurotransmitter systems which regulate mood, cognition, sleep, and appetite. Women with PMDD can have low estrogen levels in the luteal phase of their cycle, which decreases the effects of serotonin, leaving PMDD sufferers more sensitive to estrogen and progesterone fluctuations. This again highlights the complex interaction of ovarian hormones, neurotransmitters, and mood (3).
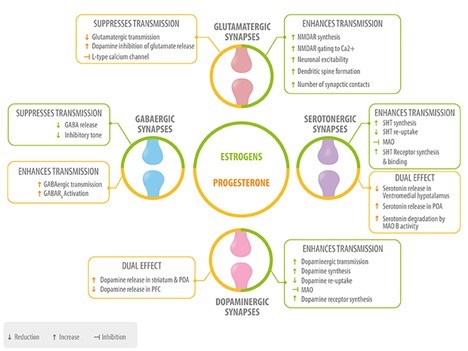
Figure 3. Role of neurosteroids in the modulation of the four main neurotransmitters. Estrogen (green) and progesterone (yellow) interact with GABAergic, glutamatergic, serotonergic, and dopaminergic synapses at different levels: neurotransmitter synthesis, release, degradation, and neurotransmitter receptor synthesis, activation or inhibition 5HT, serotonin; MAO, monoamino oxidase; POA, preoptic area; PFC, prefrontal cortex.
Thyroid function and PMDD
Subclinical thyroid disorders are abundant amongst women with menstrual, depressive, and fertility disorders. Hypothyroidism is commonly associated with depression, dysphoria, and cognitive decline while hyperthyroidism can be associated with agitation, acute psychosis, and apathy. Thyroid hormone and its receptors are abundant within the central nervous system modulating neurotransmission and exerting some influence over serotonin and norepinephrine which both have extensive effects on mood and cognitive function (9).
A 2021 pilot study out of India evaluated the correlation between thyroid dysfunction and PMDD. Of the 60 women in the study with PMDD, 63% were diagnosed with subclinical hypothyroidism. The study concluded that women with PMDD should be evaluated for thyroid dysfunction as it relates to ovarian hormone output and imbalances. Addressing thyroid dysfunction is a modifiable endocrine factor that can be easily addressed and may contribute to improvement in PMDD symptoms (9).
HPA axis, cortisol, stress, and PMDD
Along with the core mood symptoms of PMDD, women also experience increased sensitivity to stress during the luteal phase. This includes not only greater subjective perceived stress but also an altered physiologic stress response from the hypothalamic-pituitary-adrenal (HPA) axis (11). Given that cyclical ovarian hormones and their metabolites interact with the HPA axis, a history of chronic stress and adversity may contribute to the development of PMDD and increase premenstrual symptom severity (12).
Ko et al measured multiple markers in their study of 58 women with PMDD against 50 controls. Ultimately their findings revealed that women with PMDD have higher luteal phase progesterone and cortisol, and lower BDNF and VEGF which are noted to be protective against stress and promote neurogenesis and neuroplasticity (7). Progesterone can be a precursor hormone to cortisol so it may follow that higher progesterone during the luteal phase of the menstrual cycle might lead to a higher level of cortisol in response to stress.
In a 2019 article in The Annual Review of Clinical Psychology, Albert and Newhouse review the interactions of estrogen and stress in relation to depression. Major depressive disorder (MDD) can be characterized by HPA axis dysregulation. It has been demonstrated that during phases of low estrogen, women with MDD show greater negative mood and less hippocampal activity during acute stress than they do during phases of high estrogen. They conclude that estrogen may support an efficient and dynamic stress response through supporting neuroplasticity, cognitive function, serotonin, and norepinephrine (2).
Chronically elevated cortisol levels are associated with depression and structural changes within the brain. How the brain perceives stress is regulated by ventral and dorsal systems within the brain. The ventral system allows for quick appraisal of emotionally charged stimuli and includes the amygdala which participates in the regulation of autonomic and endocrine functions, including activation of the fight-or-flight response. The dorsal system includes the hippocampus that is involved in memory, learning, and emotion. The dorsal system allows for secondary appraisal of stressful stimuli modulating the emotional, physiological, and cognitive response of the more rapidly responsive ventral system (2).
Albert et al propose that mood dysregulation is the result of an imbalance in the functional activity of the ventral and dorsal systems and is mediated by hormonal fluctuations experienced across the menstrual cycle. In women, the cortisol response to stress is decreased during the phases of the menstrual cycle when estrogen is high. Estrogen enhances the response of the dorsal system that allows for a more integrated assessment of stressful stimuli (2).
PMDD treatment options
- Selective Serotonin Reuptake Inhibitors (SSRIs) – Considered the gold standard for PMDD, SSRIs can be dosed continuously or exclusively in the luteal phase. Unlike other depressive and anxiety disorders, the effect on symptoms of PMDD is rapid and requires relatively low doses (13).
- Inhibition of Ovulation - Therapies that inhibit ovulation and luteal phase hormone fluctuation include:
- Combined Oral Contraceptives (COC) - COCs have proven effective for somatic symptoms of PMDD but show inconsistent results on affective symptoms and must be dosed continuously without the use of placebo pills during menstruation (13).
- Gonadotrophin Releasing Hormone (GnRH) Receptor Agonists - GnRH receptor agonists act to suppress ovulation through down-regulation of GnRH receptors but inhibit the production of estrogen and progesterone altogether and induce menopausal symptoms (13).
- Estradiol Patch and Progestogen – Continuous use of a low-dose estradiol patch and cyclical progestogen or the use of a Mirena IUD has been presented as an option for the treatment of PMDD (14, 15).
- High Dose Progesterone - PMDD symptoms are often experienced when progesterone and ALLO are declining. PMDD symptom severity is related to ALLO serum concentration in an inverted U-shaped curve indicating that low or high concentrations of ALLO can have a positive effect on mood (3).
- Botanicals
- Vitex Agnus-Castus (Chasteberry) reduces prolactin secretion which increases the chance of ovulation and formation of the corpus luteum allowing adequate production of progesterone. Vitex also increases dopamine transmission and activates estrogen and opioid receptors (13, 16).
- Hypericum perforatum (St. John’s wort) is an antidepressant and anxiolytic that acts as a reuptake inhibitor of serotonin, dopamine, and norepinephrine (13, 17).
- Nutrients – Vitamin B6 is included among the first-line therapies for PMDD due to its function as a cofactor in the synthesis of monoamines (serotonin, dopamine, epinephrine, norepinephrine) and GABA. The additional use of thiamine, calcium, zinc, magnesium, nutritional lithium, vitamin D, fish oil, and evening primrose oil have all been cited in the literature as having some degree of positive effect (13, 18).
- Therapies – Cognitive Behavioral Therapy (CBT) as a psychotherapeutic modality has proven effective in the treatment of PMDD. Acupuncture, acupressure, massage, yoga, Epsom salt baths, and regular meditation all serve to calm the nervous system and reduce symptoms associated with PMDD (13).
- Lifestyle – High-quality, whole food diet along with regular exercise and 7-9 hours of quality sleep supports a healthy lifestyle and provides a solid foundation of good health that can only be supportive of reducing PMDD symptoms.
Putting it all together
In addition to the reproductive role that sex hormones play, they also have profound effects throughout the brain and body. While hormonal fluctuations are necessary to create the menstrual cycle, those same fluctuations are experienced within the brain and the various tissues throughout the body that also respond to these hormones. The contributing factors that lead to PMDD create a complex web of interactions that may need to be addressed at multiple levels with an individualized approach. I am inclined to believe that the presence of adequate estrogen in the luteal phase has a stabilizing influence on the effects of progesterone and ALLO on brain function and mood. This goes back to the notion that a balanced level of both hormones is necessary to create a healthy cycle.
It is always interesting to get feedback from women who have found a way to successfully manage their PMDD symptoms. In reviewing various online articles on PMDD that allowed for public comment, it is clear that some women do well with progesterone, some do well with estrogen, some do well with both, some benefit by using cyclical SSRIs, some benefit by treating thyroid dysfunction, and some do well with a combination of all or some of these modalities. Perhaps as we learn more about the causative factors contributing to PMDD, we may discover that there are two or more subtypes that require unique and specific interventions.
ZRT Testing – revealing the potential contributors to PMDD
The personal and unique history of every woman who experiences this disorder can offer clues to the cause. Treatment of PMDD is clearly not a one-size-fits-all proposition and may involve a journey of trial and error before improvement. To increase the chances of improving symptoms, testing sex hormones, thyroid function, adrenal hormones, and neurotransmitter levels may point us in the right direction.
ZRT offers menstrual cycle mapping through dried urine testing which allows us to see the rise and fall of estrogen, progesterone, and luteinizing hormone from the mid-follicular phase through the late luteal phase of the menstrual cycle. This allows us to see not only the direct measurement of estrogen and progesterone but also the relationship between them. Thyroid hormones and antibodies can be conveniently measured in dried blood spot and cortisol levels are measured in a multi-point salivary test to capture the cortisol rhythm during the waking hours. Neurotransmitter testing is also available through dried urine testing which can be combined with single day dried urine hormone measurements that include estradiol and the progesterone metabolites of pregnanediol and allopregnanolone.
References
- Mishra S, Elliott H, Marwaha R. Premenstrual Dysphoric Disorder. [Updated 2023 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
-
Albert, Kimberly M., and Paul A. Newhouse. “Estrogen, Stress, and Depression: Cognitive and Biological Interactions.” Annual Review of Clinical Psychology, vol. 15, no. 1, May 2019, pp. 399–423.
- Barth, Claudia, et al. “Sex Hormones Affect Neurotransmitters and Shape the Adult Female Brain during Hormonal Transition Periods.” Frontiers in Neuroscience, vol. 9, Feb. 2015, p. 37.
-
Norman, Anthony W., and Helen L. Henry. Hormones. 3rd ed, Elsevier, 2015. (Textbook)
-
John A Holt, Frank M. Wittmaack, James R. Schreiber, Dipak K. Ghosh, K. M. J. Menon, Estrogen Increases Precursor for Pregnenolone Synthesis with Temperature-Sensitive Occupancy of P-450scc in Mitochondria of Rabbit Corpus Luteum, Endocrinology, Volume 122, Issue 5, 1 May 1988, Pages 1948–1957.
- Yen, Ju-Yu, et al. “Early- and Late-Luteal-Phase Estrogen and Progesterone Levels of Women with Premenstrual Dysphoric Disorder.” International Journal of Environmental Research and Public Health, vol. 16, no. 22, Nov. 2019, p. 4352.
-
Ko, Chih-Hung, et al. “Estrogen, Progesterone, Cortisol, Brain-Derived Neurotrophic Factor, and Vascular Endothelial Growth Factor during the Luteal Phase of the Menstrual Cycle in Women with Premenstrual Dysphoric Disorder.” Journal of Psychiatric Research, vol. 169, Jan. 2024, pp. 307–17.
- Huo, Liang, et al. “Risk for Premenstrual Dysphoric Disorder Is Associated with Genetic Variation in ESR1, the Estrogen Receptor Alpha Gene.” Biological Psychiatry, vol. 62, no. 8, Oct. 2007, pp. 925–33.
-
Marilu Jurado-Flores, Firas Warda, Arshag Mooradian, Pathophysiology and Clinical Features of Neuropsychiatric Manifestations of Thyroid Disease, Journal of the Endocrine Society, Volume 6, Issue 2, February 2022.
-
Parvathy, S., et al. “The Profile of Subclinical Hypothyroidism in Subjects with Premenstrual Dysphoric Disorder – A Pilot Study.” Kerala Journal of Psychiatry, vol. 34, no. 1, Feb. 2021, pp. 17–20.
- Hantsoo, Liisa, and C. Neill Epperson. “Allopregnanolone in Premenstrual Dysphoric Disorder (PMDD): Evidence for Dysregulated Sensitivity to GABA-A Receptor Modulating Neuroactive Steroids across the Menstrual Cycle.” Neurobiology of Stress, vol. 12, Feb. 2020, p. 100213.
-
Nayman, Sibel, et al. “Childhood Adversity Predicts Stronger Premenstrual Mood Worsening, Stress Appraisal and Cortisol Decrease in Women with Premenstrual Dysphoric Disorder.” Frontiers in Endocrinology, vol. 14, 2023.
- Carlini, Sara V., et al. “Management of Premenstrual Dysphoric Disorder: A Scoping Review.” International Journal of Women’s Health, vol. 14, Dec. 2022, pp. 1783–801.
- O’Brien, Shaughn, and John Studd. “Premenstrual Syndrome.” Menopause International, vol. 18, no. 2, June 2012, pp. 39–40.
- Cunningham, Joanne, et al. “Update on Research and Treatment of Premenstrual Dysphoric Disorder.” Harvard Review of Psychiatry, vol. 17, no. 2, 2009, pp. 120–37.
- Jang, Su Hee, et al. “Effects and Treatment Methods of Acupuncture and Herbal Medicine for Premenstrual Syndrome/Premenstrual Dysphoric Disorder: Systematic Review.” BMC Complementary and Alternative Medicine, vol. 14, Jan. 2014, p. 11.
- Peterson, Bahtya, and Hoang Nguyen. “St. John’s Wort.” StatPearls, StatPearls Publishing, 2024.
- ZRT Blog - Nutritional Lithium: Orchestrating Our Genes & Optimizing Our Moods by Dr. James Greenblatt 2017.