In a previous four-part series, I examined some of the main issues associated with long COVID, focusing on the central nervous system, ongoing inflammation and autoimmunity, mitochondrial dysregulation and hypothalamic-pituitary-adrenal (HPA) axis dysfunction. While the science regarding these topics is still evolving, taking a closer look at the effects of long COVID on epinephrine, norepinephrine, and cortisol will provide some insight regarding the toll that COVID can take on the autonomic nervous system (ANS) and the HPA axis.
The symptoms of long COVID can be so severe that the sufferer cannot return to their previously productive life. Some of the most debilitating symptoms are fatigue, brain fog and blood pressure issues associated with ANS dysfunction. This unfortunate combination of symptoms renders the sufferer physically and mentally incapacitated and unable to work or perform basic activities of daily living. In this article, we will take a closer look at the mechanisms of dysfunction in these conditions and how the SARS-CoV-2 virus and the COVID vaccine contribute to these conditions.
Definition of Long COVID
According to the CDC, the long-term effects of COVID can be referred to as long COVID, long-haul COVID, post-COVID condition, post-acute COVID-19, post-acute sequelae of SARS-CoV-2 infection (PASC), long-term effects of COVID, and chronic COVID. Regardless of the name, the condition is defined by a wide range of symptoms that can last weeks, months, or even years after the initial infection. The symptoms may have a waxing and waning nature and the development of the condition is not dependent upon the severity of the illness (1).
There is currently no single test to diagnose long COVID and standard blood tests, scans and x-rays may all appear normal. Long COVID presents similarly to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), which is also considered a post-viral syndrome presenting with unrelenting fatigue, brain fog, body pain, sleep issues, dizziness, and orthostatic intolerance. Similar to ME/CFS, long COVID tends to relapse after exercise (post-exertional malaise), and with physical or mental activity and stress (2). Long COVID has a significant impact on the endocrine system where many of the generalized symptoms overlap with symptoms of adrenal insufficiency.
Long COVID as a Disability – Where Have All the Workers Gone?
It is suspected that symptoms of long COVID affect approximately 30-50% of those who have been infected (3). Long COVID is considered a disability under the Americans with Disabilities Act because it can substantially limit one or more major life activities and presents with physical or mental impairment associated with past COVID infection (4). A recent article in the Journal of the American Medical Association suggests that unemployment rates are higher among those with self-reported symptoms of long COVID (5). Fortune magazine estimates two to four million members of the American workforce are unemployed due to long COVID. These numbers are based on U.S. Census Bureau data released in June 2022 (6).
Long COVID symptoms are also prevalent in those who received the COVID vaccine but have not had the virus (7). The common feature between infection with the virus and the vaccine is the spike protein. If the spike protein from the infection is a major player in the development of long COVID, we must acknowledge the potential for similar symptoms to occur in the vaccinated population who have high levels of spike protein and antibodies to the spike protein.
Function of the Autonomic Nervous System
One of the most debilitating symptoms of long COVID is orthostatic intolerance, which is associated with ANS dysfunction. The ANS is part of the peripheral nervous system that regulates involuntary physiological functions such as blood pressure, heart rate, respiratory rate, and digestion (8).
The hypothalamus is one of the main autonomic centers in the brain and may serve as the route by which SARS-CoV-2 can reach the autonomic network. The hypothalamus houses a structure called the paraventricular nucleus (PVN) that not only controls neuroendocrine and autonomic function, but also regulates the HPA axis through the release of corticotrophin-releasing hormone (CRH) that stimulates the pituitary to produce adrenocorticotropic hormone (ACTH) (8). ACTH stimulates the adrenal cortex to produce cortisol as part of the stress response.
The PVN also exerts negative feedback control over the HPA axis through the presence of cortisol receptors. There must be an adequate production of cortisol as part of the stress response to provide negative feedback to the PVN in the hypothalamus. If cortisol is low, the stress response cannot self-regulate and may result in excess production of catecholamines (epinephrine, norepinephrine) (8).
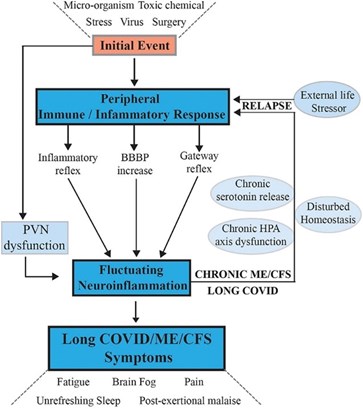
Fig 1. Signaling pathways between the central nervous system and the peripheral nervous system that maintain chronic illness with relapse and partial recovery phases. Tate W, Walker M, Sweetman E, et al. Molecular mechanisms of neuroinflammation in ME/CFS and long COVID to sustain disease and promote relapses. Front Neurol. 2022;13:87772. Open access under the Creative Commons CC-BY license.
Mechanisms of Autonomic Dysfunction Post-COVID 19
Autonomic dysfunction can present as fatigue, dizziness, fainting, shortness of breath, orthostatic intolerance, nausea, vomiting, and heart palpitations. Direct infection of the hypothalamus by the SARS-CoV-2 virus via neuronal or hematogenous routes can lead to autoantibodies against brain tissue, cause persistent inflammation, and contribute to hypoxia (8).
The hypothalamic response to infection can lead to sympathetic overactivation and increased release of epinephrine and norepinephrine. The dysfunctional ANS presentation seen in long COVID may be due to the excessive release of catecholamines in response to orthostatic intolerance and brain hypoxia. Autonomic dysfunction can occur as a presyncope or syncope episode, both of which are caused by decreased blood flow to the brain. Syncope is the medical term for fainting and usually occurs from a vasovagal, situational, or carotid sinus event. Presyncope and syncope can occur with orthostatic hypotension and postural orthostatic tachycardia syndrome (POTS). Autonomic dysfunction can also present as hypertension and cardiac arrythmias through sympathetic nervous system (SNS) hyperactivation and the resulting elevated catecholamine levels. The SNS activation occurs as a corrective response to orthostatic hypotension (8).
Low blood pressure and low blood volume activate the SNS to create high levels of catecholamines. The overactivation of the SNS may trigger an accentuated counter-regulatory response through activation of the vagus nerve resulting in paradoxical vasodilation and sympathetic withdrawal, which clinically presents as dizziness, hypotension, and ultimately presyncope or syncope (9).
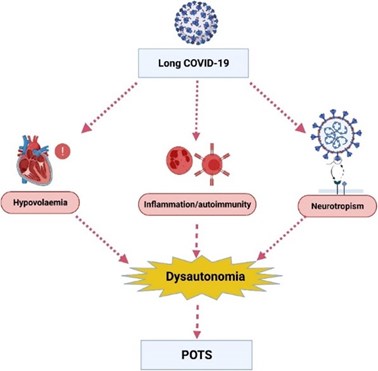
Fig 2. Potential underlying mechanisms by which long COVID leads to dysautonomia and postural orthostatic tachycardia syndrome (POTS). Chadd KR, Blakey EE, Huang C, et al. Long COVID -19 and postural orthostatic tachycardia syndrome – is dysautonomia to be blamed?
Front Cardiovasc Med. 2022;9:860198. Open access under the Creative Commons CC-BY license.
Symptoms of Autonomic Dysfunction
Orthostatic hypotension is defined as a drop in systolic blood pressure by 20 mmHg and a drop in diastolic blood pressure by 10 mmHg within three minutes of standing from a supine position. The same symptoms occur with POTS, but it is accompanied by tachycardia with a heart rate reaching at least 100 bpm within three minutes of standing. People with POTS can also experience impaired attention, processing speed and executive function, which presents as the common descriptor of brain fog. Though not proven, it is suspected that these symptoms occur due to decreased blood flow to the brain and elevated levels of norepinephrine (8).
In a study referenced by Jammoul et al, nine patients with long COVID experienced reduced cerebral blood flow upon standing, which was comparable to a group of patients with a diagnosis of POTS. The study participants also presented with symptoms of high epinephrine. Additional symptoms included diarrhea, face flushing, restlessness, and tremors. As previously mentioned, the activation of the SNS can be accentuated and ongoing if low cortisol cannot provide the negative feedback to reset the stress response and turn off the production of catecholamines (8).
HPA Axis Dysfunction and Low Cortisol Post-COVID
Hypocortisolism is the main hormonal disorder diagnosed in patients with long COVID. Central adrenal insufficiency is also described in patients with active SARS-CoV-2 infection. ACTH shares similar amino acid sequences with the SARS-CoV-2 virus, which may result in antibody production against ACTH in response to the presence of the virus (10).
Molecular mimicry is one of the leading mechanisms by which infectious or chemical agents may induce autoimmunity. It occurs when similarities between foreign and self-peptides favor an activation of autoreactive immune cells in a susceptible individual. As a result of molecular mimicry, the antibodies targeted for the virus may inactivate endogenous ACTH and destroy ACTH-secreting cells in the pituitary (2). ACTH is necessary to stimulate the adrenal cortex to produce cortisol.
The adrenal cortex also produces angiotensin-converting enzyme 2 (ACE2) receptors in the zona fasciculata and the zona reticularis. Binding of the virus to the adrenal ACE2 receptors may lead to direct damage to cells within the adrenal cortex, resulting in reduced cortisol production. Autopsy studies in patients with COVID-19 revealed necrosis of the adrenal cortical cells and identified the virus in the adrenal glands (2).
Susceptible cells in the adrenals also need to express co-receptors required for SARS-CoV-2 internalization such as transmembrane serine protease 2 and furin protease. Several reports have confirmed the expression of these receptors in the adrenal glands, indicating that the virus may actively replicate in adrenocortical cells (11). Active infection of adrenocortical cells with SARS-CoV-2 is also associated with inflammation and activation of apoptosis. Treatment of COVID with high-dose, very potent synthetic steroids may also suppress endogenous cortisol production (12).
Some studies have addressed the concern of adrenal insufficiency with long COVID. Sara Bedrose, MD, MSc, an adrenal endocrine specialist at Baylor College of Medicine, has noted that a large percentage of COVID survivors experience suboptimal cortisol secretion during ACTH stimulation testing, which is diagnostic of central adrenal insufficiency. It is suspected that the adrenal insufficiency may in part be due to pituitary gland inflammation or direct hypothalamic damage from infection (13).
Small Fiber Neuropathy and Mast Cell Activation Syndrome
Other conditions that contribute to autonomic dysfunction and have been linked to the development of long COVID are small fiber neuropathy (SFN) and mast cell activation syndrome (MCAS). Both conditions can stimulate the stress response via the SNS and the HPA axis as described above. General symptoms of SFN include fatigue, cognitive disturbances, headache, and widespread musculoskeletal pain (14). SFN has been associated with ME/CFS and has been diagnosed as a contributing disorder in the symptom profile of ME/CFS, fibromyalgia, long COVID and as a side effect of the SARS-CoV-2 mRNA vaccine.
In a July 2022 article in the Journal of Family Medicine and Primary Care, Josef Finsterer cites three cases of post-vaccine SFN. The collection of symptoms in all three women included fatigue, dizziness, flushing, palpitations, diarrhea, muscle weakness, gait disturbance, balance problems, brain fog, dysphagia, sleep problems, presyncopal sensations, hair loss, chest pain, dyspnea, paresthesias, irregular menstrual cycles, and hives. All three patients underwent a skin biopsy confirming SFN, which revealed reduced intraepidermal nerve fiber density (7). None of the patients had been diagnosed with COVID prior to their vaccinations and had uneventful medical histories. It was presumed that the SFN was immune mediated as it responded well to intravenous immunoglobulin therapy.
MCAS occurs when excessive amounts of inflammatory mediators (cytokines, histamine, heparin, growth factors) are released in response to triggers such as foods, fragrances, stress, exercise, medications, or temperature changes. Excessive release of mast cell mediators can also occur during acute and chronic infections as mast cells participate in innate and adaptive immunity. Increased activation of aberrant mast cells induced by SARS-CoV-2 infection by various mechanisms may underlie part of the pathophysiology of long COVID (15). Symptoms of MCAS include episodes of abdominal pain, cramping, diarrhea, flushing, itching, wheezing, coughing, fatigue, body pain, fainting, rapid pulse, and low blood pressure.
SFN and MCAS both have effects on vascular function, which can present as orthostatic intolerance and autonomic dysfunction. These conditions can trigger the SNS and HPA axis to release catecholamines and cortisol to regulate blood pressure and vascular dynamics. If cortisol output is low due to adrenal insufficiency, the initial response of the SNS via catecholamines may not receive adequate feedback from cortisol to decrease the output of catecholamines. Continuous activation of the HPA axis can also result in excessive release of CRH, which further stimulates mast cells (3).
The New Frontier
We are over three years into the pandemic-response-sequelae of COVID-19, and it’s not done with us yet. While the severity of the acute infection with the virus has waned, coronaviruses tend to mutate quickly so keeping up with emerging variants via vaccine protection seems to fall into the realm of “chasing our tails.” We are also faced with the somewhat daunting task of learning how to address long COVID and spike protein illness that can arise from either the virus or the vaccine. Focusing on the effects of the spike protein on different systems within the body will guide our way through this process.
The effects of the spike protein on the adrenals and the broader HPA axis has been the focus of this discussion. SARS-CoV-2 can have direct impact on the adrenals themselves and, through dysfunction in other systems, affect the overall functional response of the stress mechanisms that stimulate the HPA axis. The suspicion of adrenal insufficiency can easily be validated through timed measurements of salivary cortisol and DHEA-S. In addition, diurnal measurements of urinary cortisol, cortisone, epinephrine and norepinephrine provide insight regarding the stimulation of the SNS. Measuring cortisol and catecholamines can encompass the major players in the stress response loop. While we always must consider the underlying drivers of an imbalanced stress response, we can appropriately support the HPA axis while we attempt to address larger issues.
Recommended Testing for Long COVID
ZRT Laboratory has developed tests to evaluate response of the HPA axis and peripheral SNS in patients with long COVID syndrome. Research studies have shown that long COVID symptoms are closely associated with disrupted circadian rhythms and abnormal levels of stress hormones. Cortisol, cortisone, melatonin, norepinephrine, and epinephrine are biomarkers of the stress response to inflammation from viruses like SARS-CoV-2 and the aftermath of the disease that cause long COVID. The five stress markers are tested by LC-MS/MS in urine collected four times throughout the day (first morning, second late morning, late afternoon, and night), allowing for diurnal assessment of their levels as it relates to disease status or recovery from viral infections like COVID.
The five stress markers are stabilized by collecting and drying the urine on filter strips, which prevents degradation of norepinephrine and epinephrine, which occurs rapidly in liquid urine. Dried urine strips allow for convenient shipment to the testing lab at ambient temperature, avoiding costly and inconvenient cold chain shipment as required for liquid urine samples. Test results for each of the five stress hormones, analyzed at the four time points, are presented on real time 24-hour contiguous graphs compared with expected reference ranges in healthy individuals. This test is an excellent tool to evaluate the stress impact of long COVID, as well as other inflammatory diseases such as cardiovascular disease, cancer, diabetes, and senile dementia on adrenal and SNS function. For more information about ZRT’s Diurnal Urinary Stress Hormone Test (DUSHT), give our Customer Service Team a call at 866.600.1636.
Acronym key
Adrenocorticotropic Hormone (ACTH)
Angiotensin-Converting Enzyme 2 (ACE2)
Autonomic Nervous System (ANS)
Corticotrophin-Releasing Hormone (CRH)
Diurnal Urinary Stress Hormone Test (DUSHT)
Hypothalamic-Pituitary-Adrenal (HPA)
Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
Mast Cell Activation Syndrome (MCAS)
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Paraventricular Nucleus (PVN)
Post-Acute Sequelae of SARS-CoV-2 Infection (PASC)
Postural Orthostatic Tachycardia Syndrome (POTS)
Small Fiber Neuropathy (SFN)
Sympathetic Nervous System (SNS)
References
- Long COVID or Post-COVID conditions. Centers for Disease Control and Prevention. December 16, 2022.
- Salzano C, Saracino G, Cardillo G. Possible adrenal involvement in long COVID syndrome. Medicina (Kaunas). 2021;57(10):1087.
- Theoharides TC. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol Neurobiol. 2022;59(3):1850-1861.
- Guidance on “long COVID” as a disability under the ADA, Section 504, and Section 1557. U.S. Department of Health and Human Services. July 26, 2021.
- Suran M. Long COVID linked with unemployment in new analysis. 2023;329(9):701-702.
- Berger C. Where have all the workers gone? Long COVID has forced as many as 4 million people out of the workforce. Fortune Well. August 25, 2022.
- Finsterer J. Small fiber neuropathy as a complication of SARS-CoV-2 vaccinations. J Family Med Prim Care. 2022;11(7):4071-4073.
- Jammoul M, Naddour J, Madi A, et al. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton Neurosci. 2023;245:103071.
- Dani M, Dirksen A, Taraborelli A, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63-67.
- Normatov MG, Karev VE, Kologov AV, et al. Post-COVID endocrine disorders: putative role of molecular mimicry and some pathomorphological correlates. 2023;13(3):522.
- Kanczkowski W, Beuschlein F, Bornstein SR. Is there a role for the adrenal glands in long COVID? Nat Rev Endocrinol. 2022;18(8):451-452.
- Leow MK, Kwek DS, Ng AW, et al. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf). 2005;63(2):197-202.
- DeBakey ME. How does COVID-19 impact the adrenal gland? Baylor College of Medicine Blog Network. April 22, 2021.
- Cascio MA, Mukhdomi T. Small fiber neuropathy. StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing. 2022.
- Weinstock LB, Brook JB, Walter AS, et al. Mast cell activation symptoms are prevalent in long-COVID. Int J Infect Dis. 2021;112:217-226.